what is the change in internal energy of the system
6.3: The First Law of Thermodynamics: Internal Energy
- Page ID
- 98556
Learning Objectives
- To summate changes in internal energy
To written report the flow of energy during a chemical reaction, we need to distinguish between a arrangement, the small, well-defined office of the universe in which we are interested (such as a chemic reaction), and its surroundings, the rest of the universe, including the container in which the reaction is carried out (Figure \(\PageIndex{ane}\)). In the give-and-take that follows, the mixture of chemical substances that undergoes a reaction is ever the organisation, and the flow of heat can be from the system to the environment or vice versa.
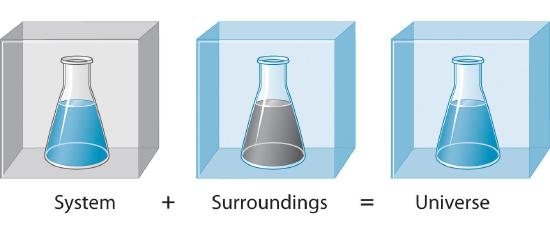
Iii kinds of systems are of import in chemistry. An open system can commutation both matter and energy with its surroundings. A pot of humid water is an open up system considering a burner supplies energy in the form of heat, and matter in the form of water vapor is lost as the h2o boils. A closed organisation can substitution energy but not thing with its environment. The sealed pouch of a ready-made dinner that is dropped into a pot of boiling water is a closed organization because thermal energy is transferred to the system from the boiling water but no thing is exchanged (unless the pouch leaks, in which case it is no longer a airtight system). An isolated organization exchanges neither free energy nor matter with the surroundings. Energy is always exchanged betwixt a system and its surroundings, although this procedure may take identify very slowly. A truly isolated system does not actually exist. An insulated thermos containing hot java approximates an isolated system, but eventually the java cools as oestrus is transferred to the surroundings. In all cases, the amount of rut lost past a system is equal to the amount of heat gained by its surroundings and vice versa. That is, the total free energy of a system plus its surround is constant, which must be truthful if free energy is conserved.
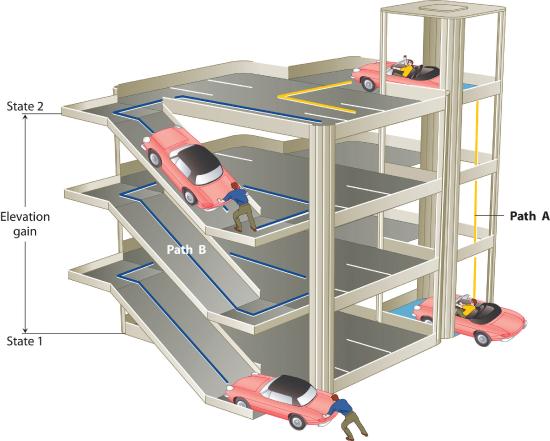
The state of a system is a complete clarification of a system at a given time, including its temperature and pressure, the amount of matter it contains, its chemical limerick, and the physical land of the thing. A state function is a property of a system whose magnitude depends on but the present state of the arrangement, non its previous history. Temperature, pressure, volume, and potential energy are all state functions. The temperature of an oven, for example, is independent of however many steps it may have taken for it to reach that temperature. Similarly, the force per unit area in a tire is independent of how oft air is pumped into the tire for it to reach that pressure, as is the terminal book of air in the tire. Heat and piece of work, on the other hand, are not country functions because they are path dependent. For case, a car sitting on the top level of a parking garage has the aforementioned potential free energy whether information technology was lifted by a crane, set there past a helicopter, driven up, or pushed up past a group of students (Figure \(\PageIndex{2}\)). The corporeality of work expended to get it there, nonetheless, can differ greatly depending on the path chosen. If the students decided to deport the machine to the superlative of the ramp, they would perform a dandy deal more piece of work than if they simply pushed the automobile up the ramp (unless, of class, they neglected to release the parking brake, in which case the work expended would increase substantially!). The potential energy of the car is the same, however, no matter which path they cull.
Direction of Estrus Flow
The reaction of powdered aluminum with iron(Three) oxide, known as the thermite reaction, generates an enormous amount of heat—enough, in fact, to cook steel (Effigy \(\PageIndex{3}\)). The balanced chemical equation for the reaction is every bit follows:
\[\ce{ 2Al(due south) + Fe_2O_3(due south) -> 2Fe(due south) + Al_2O_3(southward)} \label{5.2.1}\]
We can also write this chemical equation as
\[\ce{2Al(s) + Fe_2O_3(s) \rightarrow 2Fe(s) + Al_2O_3(s)} + \text{heat} \label{v.2.2}\]
to betoken that oestrus is one of the products. Chemical equations in which heat is shown every bit either a reactant or a product are called thermochemical equations. In this reaction, the system consists of aluminum, iron, and oxygen atoms; everything else, including the container, makes upward the environs. During the reaction, and so much heat is produced that the atomic number 26 liquefies. Eventually, the organization cools; the fe solidifies equally rut is transferred to the surroundings. A procedure in which heat (q) is transferred from a system to its surround is described as exothermic. Past convention, \(q < 0\) for an exothermic reaction.
When you lot concur an ice cube in your hand, heat from the surroundings (including your manus) is transferred to the system (the water ice), causing the ice to cook and your manus to become cold. We can depict this procedure by the following thermochemical equation:
\[ \ce{heat + H_2O(s) \rightarrow H_2O(50)} \label{v.2.3}\]
When estrus is transferred to a system from its surround, the process is endothermic. By convention, \(q > 0\) for an endothermic reaction.
Rut is technically not a component in Chemical Reactions
Technically, it is poor grade to take a \(heat\) term in the chemical reaction like in Equations \(\ref{5.2.ii}\) and \(\ref{5.2.3}\) since is it not a true species in the reaction. However, this is a user-friendly approach to represent exothermic and endothermic beliefs and is commonly used past chemists.
The Kickoff Police force
The relationship between the energy change of a organization and that of its surroundings is given by the first police force of thermodynamics, which states that the energy of the universe is constant. We can express this police mathematically as follows:
\[U_{univ}=ΔU_{sys}+ΔU_{surr}=0 \label{5.2.4a}\]
\[\Delta{U_{sys}}=−ΔU_{surr} \characterization{5.ii.4b}\]
where the subscripts univ, sys, and surr refer to the universe, the system, and the surroundings, respectively. Thus the modify in energy of a organisation is identical in magnitude but opposite in sign to the change in energy of its surroundings.
The tendency of all systems, chemical or otherwise, is to motion toward the state with the everyman possible energy.
An of import factor that determines the consequence of a chemical reaction is the tendency of all systems, chemical or otherwise, to move toward the lowest possible overall free energy state. As a brick dropped from a rooftop falls, its potential energy is converted to kinetic energy; when it reaches footing level, it has accomplished a state of lower potential free energy. Anyone nearby will notice that energy is transferred to the surroundings equally the noise of the impact reverberates and the dust rises when the brick hits the basis. Similarly, if a spark ignites a mixture of isooctane and oxygen in an internal combustion engine, carbon dioxide and h2o grade spontaneously, while potential energy (in the form of the relative positions of atoms in the molecules) is released to the surround every bit rut and piece of work. The internal free energy content of the \(CO_2/H_2O\) product mixture is less than that of the isooctane/ \(O_2\) reactant mixture. The two cases differ, however, in the form in which the free energy is released to the surroundings. In the case of the falling brick, the energy is transferred as work done on whatever happens to exist in the path of the brick; in the case of burning isooctane, the energy can exist released every bit solely estrus (if the reaction is carried out in an open container) or equally a mixture of heat and work (if the reaction is carried out in the cylinder of an internal combustion engine). Because heat and work are the only 2 means in which energy tin be transferred betwixt a organization and its surroundings, any change in the internal energy of the system is the sum of the oestrus transferred (q) and the work done (w):
\[ΔU_{sys} = q + west \label{five.two.5}\]
Although \(q\) and \(w\) are non state functions on their own, their sum (\(ΔU_{sys}\)) is independent of the path taken and is therefore a land office. A major task for the designers of any machine that converts free energy to piece of work is to maximize the amount of work obtained and minimize the amount of energy released to the environment as estrus. An instance is the combustion of coal to produce electricity. Although the maximum amount of energy bachelor from the process is stock-still by the energy content of the reactants and the products, the fraction of that energy that can be used to perform useful piece of work is not stock-still.
Considering we focus almost exclusively on the changes in the free energy of a organization, we volition non employ "sys" equally a subscript unless we need to distinguish explicitly between a system and its surroundings.
Although \(q\) and \(w\) are not state functions, their sum (\(ΔU_{sys}\)) is independent of the path taken and therefore is a country function.
Thus, considering of the offset law, we tin can determine \(ΔU\) for whatsoever procedure if nosotros tin can measure both \(q\) and \(west\). Rut, \(q\), may exist calculated past measuring a modify in temperature of the surround. Work, \(due west\), may come in different forms, only information technology as well can be measured. One important form of work for chemical science is pressure-volume piece of work done by an expanding gas. At a constant external pressure (for example, atmospheric pressure)
\[west = −PΔV \label{5.2.6}\]
The negative sign associated with \(PV\) work done indicates that the system loses energy when the volume increases. That is, an expanding gas does work on its surroundings, while a gas that is compressed has piece of work done on it by the surroundings.
Case \(\PageIndex{i}\)
A sample of an ideal gas in the cylinder of an engine is compressed from 400 mL to l.0 mL during the compression stroke against a constant pressure of 8.00 atm. At the aforementioned time, 140 J of energy is transferred from the gas to the surroundings as heat. What is the total modify in the internal energy (ΔU) of the gas in joules?
Given: initial volume, final volume, external pressure, and quantity of energy transferred as heat
Asked for: total change in internal energy
Strategy:
- Determine the sign of \(q\) to use in Equation \(\ref{5.2.5}\).
- From Equation \(\ref{5.2.6}\) calculate \(w\) from the values given. Substitute this value into Equation \(\ref{5.2.5}\) to summate \(ΔU\).
Solution
A From Equation \(\ref{five.ii.5}\), we know that ΔU = q + w. We are given the magnitude of q (140 J) and need only determine its sign. Because energy is transferred from the organization (the gas) to the environment, q is negative by convention.
B Because the gas is being compressed, we know that work is existence done on the system, so \(due west\) must be positive. From Equation \(\ref{5.2.v}\),
\[w=-P_{\textrm{ext}}\Delta V=-8.00\textrm{ atm}(\textrm{0.0500 Fifty} - \textrm{0.400 L})\left(\dfrac{\textrm{101.3 J}}{\mathrm{Fifty\cdot atm}} \right)=284\textrm{ J}\]
Thus
\[\brainstorm{marshal*} ΔU &= q + westward \\[4pt] &= −140 \,J + 284\, J \\[4pt] &= 144\, J\terminate{align*}\]
In this case, although work is done on the gas, increasing its internal energy, estrus flows from the system to the surroundings, decreasing its internal free energy by 144 J. The work done and the oestrus transferred can accept contrary signs.
Exercise \(\PageIndex{1}\)
A sample of an ideal gas is allowed to expand from an initial volume of 0.200 L to a terminal volume of 3.50 50 against a constant external pressure of 0.995 atm. At the same fourth dimension, 117 J of oestrus is transferred from the environs to the gas. What is the total change in the internal energy (ΔU) of the gas in joules?
- Answer
-
−216 J
By convention (to chemists), both heat catamenia and work take a negative sign when energy is transferred from a organisation to its environment and vice versa.
Summary
In chemical science, the small function of the universe that nosotros are studying is the system, and the rest of the universe is the surroundings. Open systems can exchange both matter and energy with their surroundings, closed systems can exchange energy only not matter with their environment, and isolated systems tin can exchange neither matter nor free energy with their surround. A state function is a property of a system that depends on just its present state, not its history. A reaction or process in which heat is transferred from a system to its environs is exothermic. A reaction or process in which rut is transferred to a system from its surroundings is endothermic. The first law of thermodynamics states that the energy of the universe is abiding. The alter in the internal free energy of a system is the sum of the heat transferred and the work done. The heat flow is equal to the change in the internal energy of the system plus the PV piece of work washed. When the volume of a system is constant, changes in its internal energy can be calculated by substituting the ideal gas law into the equation for ΔU.
Source: https://chem.libretexts.org/Courses/Bellarmine_University/BU%3A_Chem_103_(Christianson)/Phase_2%3A_Chemical_Problem-Solving/6%3A_Thermochemistry/6.3%3A_The_First_Law_of_Thermodynamics%3A_Internal_Energy
Posted by: outlawdocke1945.blogspot.com


0 Response to "what is the change in internal energy of the system"
Post a Comment